
JBISRIR endorses PRISMA Statement
JBI's journal takes steps to further improve the reporting completeness of systematic reviews.
The JBI Database of Systematic Reviews and Implementation Reports (JBISRIR) is an official endorser of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statement.
The PRISMA Statement is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses. It comprises a four-phase flow diagram and 27 checklist items that pertain to the content of a systematic review and meta-analysis, which include the title, abstract, methods, results, discussion and funding.
The aim of the PRISMA Statement is to help authors improve the reporting of systematic reviews and meta-analyses. Therefore, as an endorser of the PRISMA Statement, the JBISRIR requires that authors adhere to the PRISMA checklist and use a PRISMA flow diagram as a condition of submission when reporting findings from a systematic review.
Dr Cindy Stern, Senior Associate Editor of the JBISRIR, was instrumental in the JBISRIR formally endorsing the PRISMA Statement. Dr Stern is the author of the editorial ‘Systematic review reporting: how can we do better’ which fulfils one of the steps in order to officially endorse the PRISMA Statement. She explains that by addressing each item on the checklist and including a flow chart provides for more complete reporting of systematic reviews overall.
“There is a need for a standardised approach to how systematic reviews should be reported (much like the process of conducting a systematic review), to reduce variability and increase transparency. Endorsing PRISMA is one way we can do this”, said Dr Stern.
“We hope that other journals will support PRISMA, too and help reinforce the importance of the transparent reporting of systematic reviews”, added Dr Stern.
To view the JBISRIR instructions for authors visit http://edmgr.ovid.com/jbisrir/accounts/ifauth.htm
Further reading:
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097
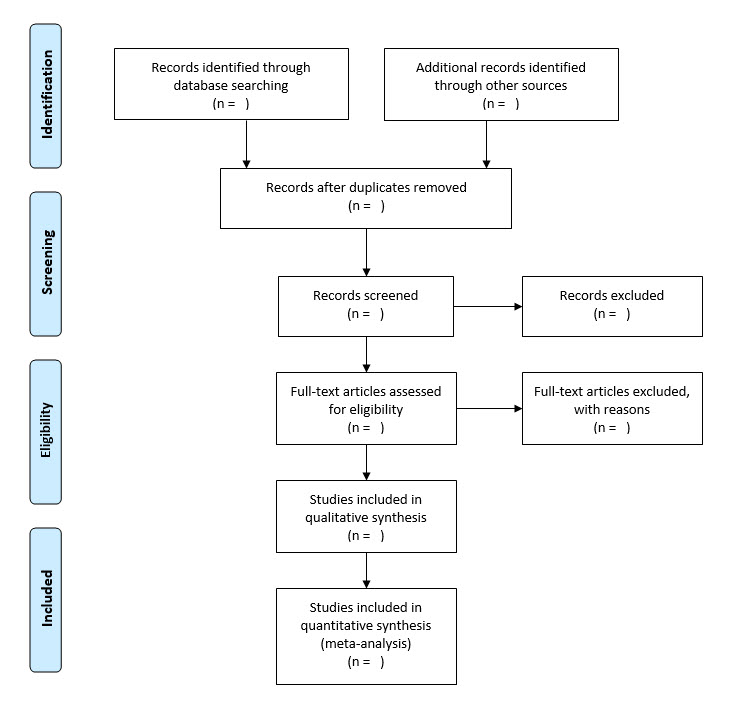
From Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097