JBI launches new short course for rapid evidence synthesis
Providing the best available evidence to inform clinical decision-making
JBI is excited to launch a new three-day short course, the Rapid Evidence Synthesis: JBI Evidence Summary Workshop.
The workshop has been developed to provide healthcare professionals with the skills required to create reliable and trustworthy JBI Evidence Summaries to support timely clinical decision-making.
What is rapid evidence synthesis?
Rapid evidence synthesis is used to provide timely, high-quality information to inform urgent decision-making by healthcare planners, providers, policymakers, etc. Rapid evidence synthesis uses systematic review methods, but streamlines synthesis by simplifying or accelerating some steps.
Who should attend?
The workshop is tailored for clinicians, researchers, administrators, academics, managers, quality improvement personnel, students, and others, interested in learning how to create evidence-based resources to support decision-making at the point-of-care, specifically JBI Evidence Summaries.
What is a JBI Evidence Summary?
JBI Evidence Summaries distill the findings from high-quality systematic reviews into concise and actionable insights for busy decision-makers. They are concise, 2–4-page documents using clear and clinician-friendly language in an easy-to-follow format to ensure easy access and rapid uptake of knowledge.
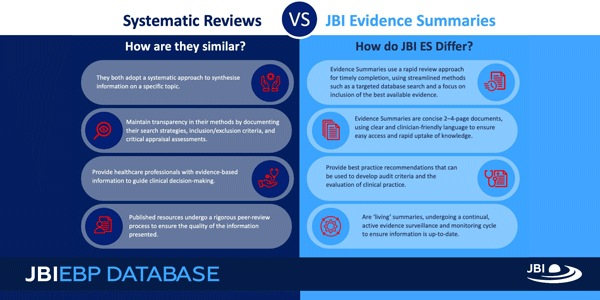
Instead of only describing what the systematic reviews say, JBI Evidence Summaries highlight the relevance, strength and applicability of the evidence to real-world practice. They also provide best practice recommendations that can be used to develop audit criteria and the evaluation of clinical practice.
Additionally, JBI Evidence Summaries are "living summaries" as they undergo a continual, active evidence surveillance and monitoring cycle to ensure information is up-to-date. Standardisation is considered a significant marker of quality and reliability, and as such JBI Evidence Summaries are developed following JBI’s standardised, rigorous evidence-based methodology.
JBI Methodology for rapid evidence synthesis
JBI’s methodology for rapid evidence synthesis stays true to core systematic review principles, including transparency, rigour, and minimising bias. JBI methodology for rapid evidence synthesis provides structured guidance on where and how steps can be adapted, while still emphasising the importance of critical appraisal and clear reporting. The focus is always on maintaining trustworthiness, even when time or resources are limited.
What can participants expect?
This workshop will provide participants with the opportunity to explore the pivotal role of evidence transfer in evidence-based practice and learn how clinical decision-support resources enhance decision-making and promote continuous quality improvement.
Participants will gain hands-on experience with the principles of developing clinical decision support resources, specifically JBI evidence summaries published in the JBI EBP Database. Through comprehensive illustrations, expert guidance by trainers, and interactive activities, participants will immerse themselves in the JBI methodology for creating impactful resources.
Course overview
The course is structured across eight modules:
Module 1: Introduction to JBI and Evidence-Based Healthcare (EBHC)
Module 2: Understanding Evidence Transfer and Clinical Decision Support Resources
Module 3: Identifying a Topic Area and Defining a Question
Module 4: Searching for the Evidence
Module 5: Selecting and Assessing the Quality of the Best Available Evidence
Module 6: Summarising and Presenting Evidence
Module 7: Developing Best Practice Recommendations and Audit Criteria
Module 8: Disseminating Findings
Workshop outcomes
By the end of the workshop, participants will have a strong foundation in knowledge translation and be equipped to create resources that support evidence-based decision-making and drive positive change in clinical practice, such as:
• Discuss EBHC concepts and the role of clinical decision support tools, such as JBI Evidence Summaries, in transferring evidence to practice
• Identify a topic area and define a question for Evidence Summary development
• Search for and select the best available evidence to include in an Evidence Summary
• Summarise and present the information effectively
• Understand the principles of developing Best Practice Recommendations and audit criteria
• Discuss the importance of dissemination
Meet your trainers!
This short course will be delivered by members of the Transfer Science team at JBI, who all bring a wealth of knowledge and experience in evidence synthesis and clinical decision support.
Find out more about your trainers:
Dr Kylie Porritt - Director, Transfer Science, JBI
Vincent Pearson - Research Fellow, Transfer Science, JBI
Dr Ashley Whitehorn - Research Fellow, Transfer Science, JBI
Dr Kirsten Macaitis - Research Fellow, Transfer Science, JBI
Bronwyn Overall - Research Fellow, Transfer Science, JBI
Dr Amy Woods - Research Fellow, Transfer Science, JBI
Dr Rian Gabor - Research Fellow, Transfer Science, JBI
Dr Matthew Stephenson - Research Fellow, Transfer Science, JBI
Make an impact
JBI’s new Rapid Evidence Synthesis: JBI Evidence Summary Workshop is one of various JBI education and training programs offered, which also include evidence synthesis and evidence implementation short courses. By participating in this workshop, participants will be better equipped to develop resources that support evidence-based decision-making and improve clinical outcomes for better patient health. Visit the JBI events page, which is regularly updated, to explore all upcoming JBI and JBI Collaboration short courses held in Adelaide and worldwide. Find a course location near you.
Additional resources
Methodological components, structure, and quality assessment tools for evidence summaries: a scoping review
Whitehorn, Ashley; Lockwood, Craig; Hu, Yan; Xing, Weijie; Zhu, Zheng; Porritt, Kylie
JBI Evidence Synthesis 23(3):p 493-516, March 2025. | DOI: 10.11124/JBIES-23-00557